The focus of audits carried out by regulatory authorities reflect the current debate of national and international legislators on the interpretation and implementation of the rules of good manufacturing practice (GMP). An analysis of the findings of the Food and Drug Administration (FDA) from the past year indicates that one primary focus is the shortcomings identified in process validation. However, the FDA repeatedly criticizes fundamental weaknesses in the qualification of production equipments.
GxP-Blog
The requirements for adequate qualification of facilities, systems and equipment as specified in Annex 15 to the GMP Guide unchanged for 15 years have developed further in the past several years. Just 10 years ago, process- and system-related issues were the focal points of «classic» equipment qualification. Today, the call for a «comprehensive, holistic» process-based approach that includes risk assessment plays a primary role (see also the Good Practice Guide «Applied Risk Management for Commissioning and Qualification», ASTM International Standard E2500 of the International Society for Pharmaceutical Engineering [ISPE]). The product and process requirements must already be known and sufficiently taken into account when defining qualification efforts. Proof of the suitability for intended use of the relevant system must be provided. The FDA guideline on process validation states that system qualification without an understanding of the actual manufacturing process cannot ensure adequate quality. The manufacturing process is thus given a more important role during the qualification of the production facilities. Standardized qualification documents that are not based on a thorough knowledge of processrisks and that do not indicate whether the future manufacturing process was taken into account will no longer suffice to document the suitability of a system for its intended use.
How can the general suitability of a system for its intended future use be proven?
A look at the rules and their interpretations shows that product and process requirements need to be understood in detail (scientifically sound understanding) in order to facilitate the definition of risk-based controls and qualification tests. Only such a set of qualification tests can ensure the suitability of the system for the intended use and thus ensure patient safety.
The suitability must be documented for the entire life cycle of a system. The ongoing qualification of a system does not end after PQ, but necessitates continuing risk analysis and repeated requalifications. Should new research results provide additional information regarding the process or product requirements, this information must be taken into account in any revision of the risk analysis and the resulting qualification tests.
Defects and shortcomings identified by a regulatory audit during system qualification that were due to a lack of understanding of the processes involved and/ or ignoring the product requirements are the primary drivers for the advances of system qualification requirements.
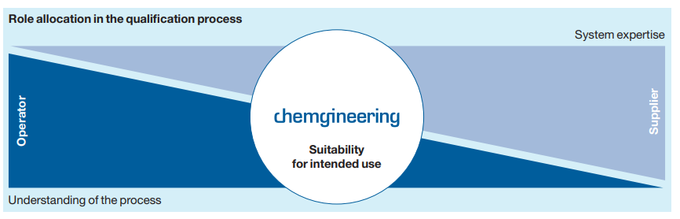
Role allocation
This paradigm shift has caused a change in role allocation between system suppliers and pharmaceutical manufacturers. While the technical expertise (in mechanics and automation) of the system suppliers previously sufficed to carry out system qualification up to OQ largely without intensive involvement of the later operators, much closer collaboration and coordination between the parties is now necessary. Many suppliers in the pharmaceutical industry offer standardized qualification packages that include all mechanical and control components of the systems. Even though these standard documents are frequently generated on a risk-based appoach, they can no longer meet the current regulatory requirements for qualification without knowledge and inclusion of the customer-specific product and process requirements.
System suppliers need more in-depth knowledge of products and processes, while the future operators must cover the technical details of their systems much more intensively long before the start of the qualification process. The engineering partner who is familiar with both the process and product requirements and possesses the necessary technical expertise with regard to the process systems now plays a primary role. He is in charge of communication between all parties and ensures that the qualification requirements are consistently and comprehensively fulfilled.
Figure 1: Teamwork among the supplier, planner and operator
Efficient qualification strategies
Our experience shows that the success or failure of a qualification project frequently depends on the interfaces between the contractor, the client and the supplier. The project organization must be adapted to the complexity of the qualification project. The qualification coordinator thereby plays a central role in complex projects. He coordinates all of the parties involved and ensures not only good communication but also compliance with the schedule. The qualification process for subsystems, package units, analysis systems, clean rooms and all other systems must be defined very early. It is important differentiate between start-up/initial commissioning tests (Good Engineer[1]ing Practice [GEP]) and qualification tests relevant to GMP. If at the beginning of the project the qualification strategy is explained to the suppliers, or if the suppliers have influence on this strategy, a high level of efficiency can be achieved. Qualification-relevant tests can be carried out by the suppliers as part of factory acceptance tests (FATs). If the supplier is familiar with the quality requirements of the customer and complies with specified acceptance criteria, time-consuming repeat tests at the future operator’s site can be omitted. Qualification can be completed faster and production started more quickly.
The respective partners must be thoroughly familiar with their roles to facilitate this. It is often the case that certain functions can only be tested when different units are working together across respective supplier boundaries. Further coordination is required at these interfaces. For example, the function of a cleaning-in-place (CIP) system can only be qualified when the installation and start-up of the systems to be cleaned and the supporting media systems have been completed and the process control system has been installed.
Change management is another essential component of an efficient qualification strategy. Handling changes and the corresponding administrative work involved tie up resources, which are then no longer available for other tasks, leading to possible delays in the project. The participating departments (quality assurance, production and qualification coordination) must define how changes are to be handled at the start of the project.
Efficient qualification strategies require matching project organization, which Chemgineering tailors to the respective customer requirements. Thus, we meet the requirements of global players as well as those of small and mid-sized pharmaceutical companies.
Chemgineering’s expertise in qualification
Chemgineering has followed the regulatory trends and supported its customers in complex regulatory environments very successfully for more than 15 years now. During this time, we provided more than 800,000 hours of qualification services to our customers. Over the past several years we have observed a strong increase in the demand for qualification expertise that has been proven in practice. Even before our specialist engineers get involved in the project, our customers can expect sound expertise in GMP compliance along with in-depth knowledge of the manufacturing processes and product requirements. The efficient qualification strategies we develop for our customers are used in the production of active substances, the manufacturing of medicinal products (sterile, solid, semi-solid) and in medical technology. Chemgineering is currently coordinating large qualification projects in Germany, Austria and Switzerland.
This website uses cookies to provide you with the best possible experience. If you continue to browse our site, you consent to our use of cookies and to our privacy policy.