The transfer of analytical methods is a topic that is greatly discussed and commonly practiced in many GMP laboratories. Prior to a method being transferred, the development and validation of the suitable analytical methods represents a critical component of the entire life cycle of drug development. The implementation of the transfer process must be carefully planned, taking into account a full assessment of all aspects of the method history (including initial development and validation) as well as any difficulties and issues that might already be known. In this blog article, I shall discuss the necessary key components of a method transfer between two laboratories and how it can be performed successfully.
GxP-Blog
Each technology transfer (process) between two laboratories involves two main parties: namely, the transferring laboratory (TL) and the receiving laboratory (RL). The transferring laboratory is the source or laboratory of origin for the analytical procedure, the RL is its recipient.
According to USP <1224>, the transfer of an analytical method is defined as the documented process that qualifies a laboratory (RL) to use an analytical method originating in another laboratory (TL), regardless of whether it is an internal or external laboratory.
To prepare the transfer of the method, the TL must provide the RM with all the relevant information. This transfer package usually includes the following:
- analytical data
- previous method validation reports
- previous transfer reports
- relevant standard operating procedures (SOPs)
- sample chromatograms
- quality control (QC) trends/stability data
- if applicable, a list of problems that have already occurred and how they were resolved.
A method transfer protocol (MTP) is created for this purpose, which is then signed by both parties (TL and RL) and includes at least the following:
- objectives of the method transfer
- the scope and responsibilities of the TL and RL
- method specifications
- test setup and acceptance criteria
- documentation (including the information to be provided with the results and any report forms that need to be used)
- references
- report approval
- details regarding reference samples (raw materials, intermediates and finished products).
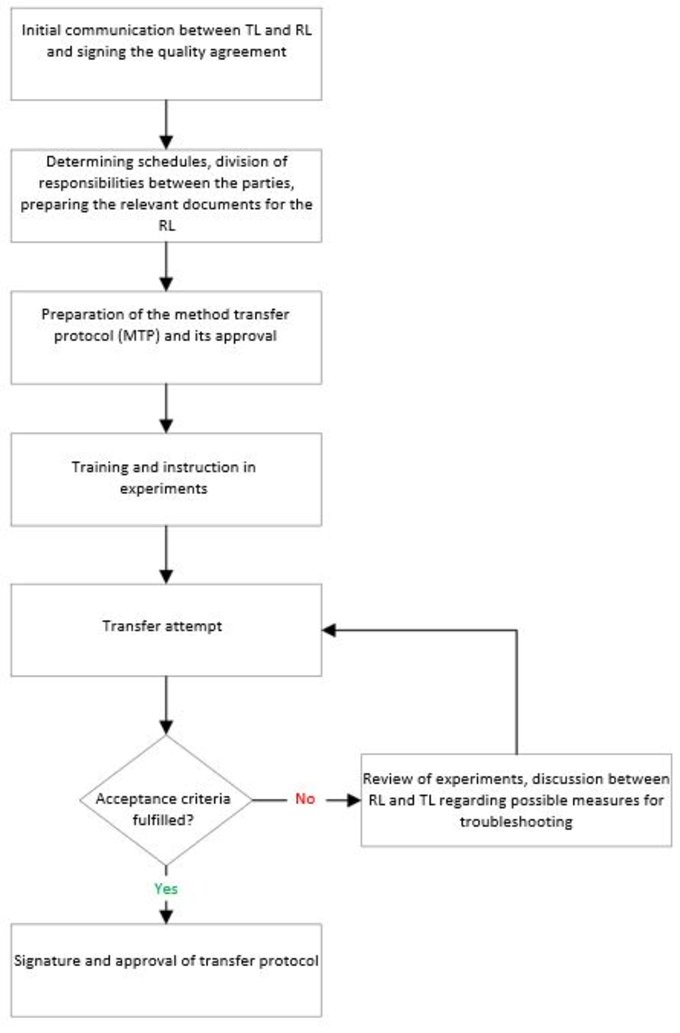
The RL then checks the MTP and provides competent – and if possible, experienced – personnel to implement the transfer process. The workflow between the two parties is described in Figure 1.
If the acceptance criteria are not fulfilled (Figure 1), the methods described in the MTP must be completely checked to ensure their validation.
Figure 1: The workflow between TL and RL
Additional aspects to review:
- Is the TL validation being carried out according to the current ICH guidelines?
- Does the validation design cover the intended use of the method (e.g. approval/stability/process test, etc.)?
- Do the results obtained by the transferring laboratory meet the acceptance criteria?
In addition to the methodological aspects, it must also be ensured that all necessary equipment, chemicals and any other specified consumables are available within the RL during the implementation period set for the method transfer.
Acceptance criteria for a successful method transfer:
Acceptance criteria are critical components of the analytical method transfer and must therefore be specifically defined.
Different approaches can be used to define acceptance criteria for the transfer, e.g. adjusting acceptance criteria for validation, knowledge of the process capability index (KPCI) or applying a statistical approach after establishing an acceptable product risk.
For less complex methods, direct comparisons of results can be made, while for complex applications statistical equivalence tests must be applied.
There are basically three ways to define the acceptance criteria for method transfers: absolute limits for differences and variability, statistical significance tests (t-tests) and statistical equivalence tests.
Method transfer strategies:
When defining the transfer strategy that needs to be applied, an assessment should be carried out regarding (1) the type of method, (2) its validated status, (3) the intended product and (4) the experience of the RL. The method transfer procedure may vary depending on the outcome of these evaluations. The specific application of the method (e.g. release, stability, in-process, etc.) must also be given full consideration.
The nature of the method, the experimental set-up and the data analysis must be tailored to the respective situation to ensure that regulatory requirements are met. A method transfer can be greatly simplified if, for example, the RL already has experience with implementing a similar method, different strengths or administration forms of the same product.
The following (Table 1) describes the different types of method transfer categories based on USP 1224:
Table 1: Different types of method transfer strategy with examples:
Conclusion/summary:
Method validation and transfer are integrated activities within analytical life cycle management. The results of the method transfer should confirm the validation status of an analytical method in a laboratory other than the validation laboratory. To ensure methodological consistency between two sites, a transfer approach and design should take the technical risks into account. Overall, a successful transfer requires the exchange of information, communication and coordination between the TL and the RL.
References
1 USP <1224> Transfer of Analytical Procedures. USP–NF. US Pharmacopeial Convention: Rockville, MD.
2 Guidance for Industry: Analytical Procedures and Methods Validation for Drugs and Biologics. US Food and Drug Administration: Silver Spring, MD, 2015.
3 ICH Q2(1): Validation of Analytical Procedures. The International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use: Geneva, Switzerland, 1994.
This website uses cookies to provide you with the best possible experience. If you continue to browse our site, you consent to our use of cookies and to our privacy policy.